Steamy topics: sex and boiling water
Today's post is largely for visitors from Michele Agnew's blog. It's got two parts and you can comment on either side, depending on which you prefer. The topics de jour are sex and science.
 The sex thing is desire. How often do you actually want to have sex? Now that sorta thing varies on all sorts of variables but still there's a base level that most people rotate around. It can go up or down depending on age, medical condition, season of the year, or more to the point--who you're sharing a bed with. LOL?
The sex thing is desire. How often do you actually want to have sex? Now that sorta thing varies on all sorts of variables but still there's a base level that most people rotate around. It can go up or down depending on age, medical condition, season of the year, or more to the point--who you're sharing a bed with. LOL?
For me, twice a week seems to be the sweet spot but I'm adaptable. Most women I've known in that way--with the notable exception of someone I shared my last name with temporarily--have been more "lively" so that adaptability has been stressed a number of times. How about you? What floats your proverbial boat?
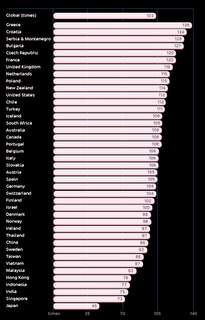
The picture at left is from some survey that compared how many times a year a person had sex with the variable being country of origin. Here's the page I got the picture from with more info on it.
*whew* Now that the nasty sex thing is out of the way we can get back to a serious topic. This time it's, of all things, boiling water. Sure, it sounds pretty mundane but I found out yesterday that it's not as widely understood as I thought.
Here's how the topic came up. There was something that would have worked out best if it was done in 5 minutes but first a critical element had to be immersed in boiling water for 10 minutes so I said that we'd have to wait. The woman I was working with suggested that I raise the temperature on the hot plate so that the reaction would go quicker so we'd be able to move onto the IHC (that's immunohistochemistry if you really want to know) portion of the experiment.
At first I thought she was kidding but it turned out she was serious. She thought that raising the temperature of a surface would increase the temperature of water that was already at a boil. I suppose in retrospect that's not all that weird an idea. After all, most things get hotter when we apply more heat. However boiling water isn't "most things". The reason for this is that there's a phase change going on at the boiling point of water.
Water has 3 phases: solid; liquid; and gas. Moving from one to another requires a change in energy. This energy varies from substance to substance but in the case of boiling water it's quite high. In fact it requires 5 times more energy to move H2O from being water into steam than it did to heat it from 32f up to a boil. This means that water resists, if you will, the phase change into steam (gas).
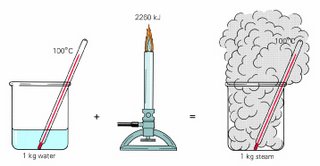 How does this have anything to do with the situation I mentioned earlier, you ask. Well, as you add more heat to water it gets hotter up until the boiling point. At that temperature it makes a phase change into steam but that requires a lot of energy so as you increase the heat the temperature of the water stays the same, the result is just an increase in the generation of steam. By the way, this is also why it's so easy to get burned by steam. The large amount of energy it takes to create steam is released when you go from steam to water. So when you inadvertently put your hand over a steaming kettle and you get beads of moisture on your hand, each droplet of water there came with a lot of energy in the form of heat. Ouch!
How does this have anything to do with the situation I mentioned earlier, you ask. Well, as you add more heat to water it gets hotter up until the boiling point. At that temperature it makes a phase change into steam but that requires a lot of energy so as you increase the heat the temperature of the water stays the same, the result is just an increase in the generation of steam. By the way, this is also why it's so easy to get burned by steam. The large amount of energy it takes to create steam is released when you go from steam to water. So when you inadvertently put your hand over a steaming kettle and you get beads of moisture on your hand, each droplet of water there came with a lot of energy in the form of heat. Ouch!
Here's a little bit about it from Wikipedia:
I find that kinda crap interesting but I suspect not many others do. I can usually put my girlfriend to sleep if I talk about stocks and monetary issues--I bet science talk will do it to a lot of folk.
 The sex thing is desire. How often do you actually want to have sex? Now that sorta thing varies on all sorts of variables but still there's a base level that most people rotate around. It can go up or down depending on age, medical condition, season of the year, or more to the point--who you're sharing a bed with. LOL?
The sex thing is desire. How often do you actually want to have sex? Now that sorta thing varies on all sorts of variables but still there's a base level that most people rotate around. It can go up or down depending on age, medical condition, season of the year, or more to the point--who you're sharing a bed with. LOL?For me, twice a week seems to be the sweet spot but I'm adaptable. Most women I've known in that way--with the notable exception of someone I shared my last name with temporarily--have been more "lively" so that adaptability has been stressed a number of times. How about you? What floats your proverbial boat?
The picture at left is from some survey that compared how many times a year a person had sex with the variable being country of origin. Here's the page I got the picture from with more info on it.
*whew* Now that the nasty sex thing is out of the way we can get back to a serious topic. This time it's, of all things, boiling water. Sure, it sounds pretty mundane but I found out yesterday that it's not as widely understood as I thought.
Here's how the topic came up. There was something that would have worked out best if it was done in 5 minutes but first a critical element had to be immersed in boiling water for 10 minutes so I said that we'd have to wait. The woman I was working with suggested that I raise the temperature on the hot plate so that the reaction would go quicker so we'd be able to move onto the IHC (that's immunohistochemistry if you really want to know) portion of the experiment.
At first I thought she was kidding but it turned out she was serious. She thought that raising the temperature of a surface would increase the temperature of water that was already at a boil. I suppose in retrospect that's not all that weird an idea. After all, most things get hotter when we apply more heat. However boiling water isn't "most things". The reason for this is that there's a phase change going on at the boiling point of water.
Water has 3 phases: solid; liquid; and gas. Moving from one to another requires a change in energy. This energy varies from substance to substance but in the case of boiling water it's quite high. In fact it requires 5 times more energy to move H2O from being water into steam than it did to heat it from 32f up to a boil. This means that water resists, if you will, the phase change into steam (gas).
 How does this have anything to do with the situation I mentioned earlier, you ask. Well, as you add more heat to water it gets hotter up until the boiling point. At that temperature it makes a phase change into steam but that requires a lot of energy so as you increase the heat the temperature of the water stays the same, the result is just an increase in the generation of steam. By the way, this is also why it's so easy to get burned by steam. The large amount of energy it takes to create steam is released when you go from steam to water. So when you inadvertently put your hand over a steaming kettle and you get beads of moisture on your hand, each droplet of water there came with a lot of energy in the form of heat. Ouch!
How does this have anything to do with the situation I mentioned earlier, you ask. Well, as you add more heat to water it gets hotter up until the boiling point. At that temperature it makes a phase change into steam but that requires a lot of energy so as you increase the heat the temperature of the water stays the same, the result is just an increase in the generation of steam. By the way, this is also why it's so easy to get burned by steam. The large amount of energy it takes to create steam is released when you go from steam to water. So when you inadvertently put your hand over a steaming kettle and you get beads of moisture on your hand, each droplet of water there came with a lot of energy in the form of heat. Ouch!Here's a little bit about it from Wikipedia:
On the other hand, the molecules in liquid water are held together by relatively strong hydrogen bonds, and its standard enthalpy change of vaporization, 40.8 kJ/mol, is more than five times the energy required to heat the same quantity of water from 0 °C to 100 °C (cp = 75.3 J K−1 mol−1).
I find that kinda crap interesting but I suspect not many others do. I can usually put my girlfriend to sleep if I talk about stocks and monetary issues--I bet science talk will do it to a lot of folk.
Comments
Is that too much? ;)
I look at it this way: We don't say "Oh I couldn't POSSIBLY have lunch, I just had it yesterday..."
Here via michele, Dave, and thanks for asking!
Mr. kenju once thought that increasing the temp on my stove would make the water boil faster and he didn't believe me when I told him it wouldn't. I will let him read this, so he will know I knew what I was talking about!!
Michele sent me this way.
As for the sex comment...well I knew there was a reason I wanted a boyfriend!! :D
Here from Michele's.
As for the boiling water, I only know that it is required to make mac n cheese!
Here via Michele's
Because I don't get to see my man all that often when we are together it's a total sex fest (multiple times per 24hr period) But I could most certainly survive on twice a week. It gets a bit tiring going at it 24/7.
xoxo Y
Here via michele again, Dave!
vegetables and reincarnation
I think I'll come back as a cucumber
Boiling water... if the experiment says something should be in boiling water for ten minutes, then ten minutes it is. You could do one for ten minutes and one for five and see if any difference turns up in the end.
Thanks for finding this, Dave. And for tackling a tricky subject. I'll take the fifth on this one: if my wife reads me commenting online, I'm sunk.
Howdy from Michele's, and from my cold house. Maybe we've got a solution to the furnace problem after all!