oxidative reduction
Sexy title, huh? Yeah. Chemistry really gets people's attention. That's why chemists get all the hot chicks. LOL Not so, you say? Well, maybe you're right. Chemistry's pretty important though--even if it's not very effective at getting dates.
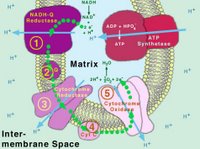 What you see to the left there is a graphic representation of cellular respiration which is a series of oxidative reductive steps which strip atmospheric oxygen of two electrons each and in turn add some hydrogen atoms to it to replace those electrons in the outer most electron shell. The resulting molecule is two hydrogens and one oxygen. H2O, also known as water.
What you see to the left there is a graphic representation of cellular respiration which is a series of oxidative reductive steps which strip atmospheric oxygen of two electrons each and in turn add some hydrogen atoms to it to replace those electrons in the outer most electron shell. The resulting molecule is two hydrogens and one oxygen. H2O, also known as water.
Why am I whining about respiration to y'all? Well, this past week a very interesting paper (okay--interesting to some people!) was published in Nature regarding this subject.
It seems that researchers at the Institute of Biotechnology of the University of Helsinki, headed by professor Marten Wikstrom, have for the first time identified an internal electron transfer reaction that initiates the proton pump mechanism of the respiratory enzyme. The respiratory enzyme cytochrome oxidase (which you can see in that picture above) functions as a proton pump that transduces free energy from oxygen reduction to an electrochemical proton gradient, which is utilized by another enzyme to produce ATP. Exciting stuff, no?
This is an area that's been studied for over 100 years. The only reason this finding took so long is that enzymes like NADH and cytochrome oxidase just work so goddamn fast. Wikstrom studied both the chemical reaction and the proton pump of cytochrome oxidase by biophysical techniques with a time resolution of less than one microsecond. That's one millionth of a second. In this way it has been possible to monitor the enzyme's functions in real time. It takes about one millisecond for the respiratory enzyme to reduce one oxygen molecule to water. This time includes all the partial reactions as well. Until now nobody was able to follow chemicals reactions like this as they happened.
Now that the technique exists, there's going to be a lot more interesting things happening in chemistry. Keep your eyes peeled for this--and it might be a good time to buy into the stock of a chemical company or two.
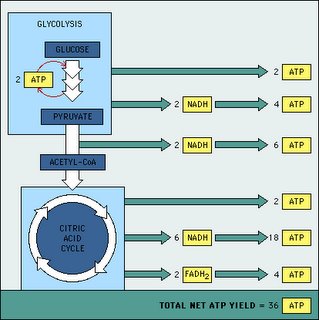 Maybe next time we can talk about why oxidative reduction is so important in respiration. At least for us animals. In case you want a hint--it's partially due to efficiency and also heat generation. The picture up above graphs out the difference between glycolysis and oxidative reduction.
Maybe next time we can talk about why oxidative reduction is so important in respiration. At least for us animals. In case you want a hint--it's partially due to efficiency and also heat generation. The picture up above graphs out the difference between glycolysis and oxidative reduction.
 What you see to the left there is a graphic representation of cellular respiration which is a series of oxidative reductive steps which strip atmospheric oxygen of two electrons each and in turn add some hydrogen atoms to it to replace those electrons in the outer most electron shell. The resulting molecule is two hydrogens and one oxygen. H2O, also known as water.
What you see to the left there is a graphic representation of cellular respiration which is a series of oxidative reductive steps which strip atmospheric oxygen of two electrons each and in turn add some hydrogen atoms to it to replace those electrons in the outer most electron shell. The resulting molecule is two hydrogens and one oxygen. H2O, also known as water.Why am I whining about respiration to y'all? Well, this past week a very interesting paper (okay--interesting to some people!) was published in Nature regarding this subject.
It seems that researchers at the Institute of Biotechnology of the University of Helsinki, headed by professor Marten Wikstrom, have for the first time identified an internal electron transfer reaction that initiates the proton pump mechanism of the respiratory enzyme. The respiratory enzyme cytochrome oxidase (which you can see in that picture above) functions as a proton pump that transduces free energy from oxygen reduction to an electrochemical proton gradient, which is utilized by another enzyme to produce ATP. Exciting stuff, no?
This is an area that's been studied for over 100 years. The only reason this finding took so long is that enzymes like NADH and cytochrome oxidase just work so goddamn fast. Wikstrom studied both the chemical reaction and the proton pump of cytochrome oxidase by biophysical techniques with a time resolution of less than one microsecond. That's one millionth of a second. In this way it has been possible to monitor the enzyme's functions in real time. It takes about one millisecond for the respiratory enzyme to reduce one oxygen molecule to water. This time includes all the partial reactions as well. Until now nobody was able to follow chemicals reactions like this as they happened.
Now that the technique exists, there's going to be a lot more interesting things happening in chemistry. Keep your eyes peeled for this--and it might be a good time to buy into the stock of a chemical company or two.
 Maybe next time we can talk about why oxidative reduction is so important in respiration. At least for us animals. In case you want a hint--it's partially due to efficiency and also heat generation. The picture up above graphs out the difference between glycolysis and oxidative reduction.
Maybe next time we can talk about why oxidative reduction is so important in respiration. At least for us animals. In case you want a hint--it's partially due to efficiency and also heat generation. The picture up above graphs out the difference between glycolysis and oxidative reduction.
Comments